Biologic Patent Protection: When Biosimilars Can Enter the U.S. Market
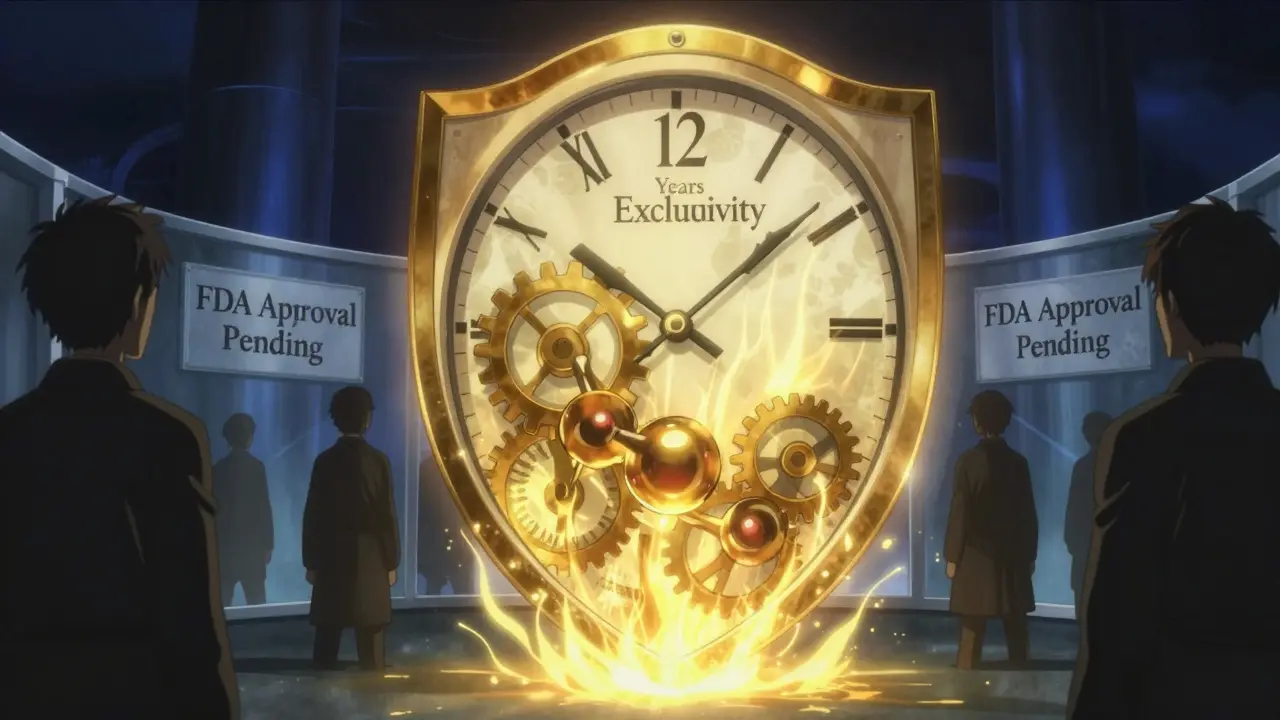
When a biologic drug hits the market, it doesn’t just come with a high price tag-it comes with a legal shield that can last over a decade. Unlike generic pills that can copy a small-molecule drug almost immediately after patent expiration, biosimilars face a much longer wait. Why? Because the rules for biologics aren’t the same. The U.S. created a special system in 2010 under the Biologics Price Competition and Innovation Act (BPCIA), and it’s designed to protect drugmakers for 12 full years before any biosimilar can even be approved.
What’s the real timeline for biosimilar entry?
The clock starts ticking the day the FDA approves the original biologic. For the first four years, no one can even file an application to make a copy. That’s called data exclusivity. After those four years, companies can submit their biosimilar applications-but the FDA still can’t approve them until 12 years have passed. So even if a company spends years developing a biosimilar, they’re stuck in regulatory limbo for eight years after filing.
That’s not a typo. It’s 12 years total. Compare that to Europe, where biologics get 10 years of data protection and just one more year of market exclusivity-11 years total. Japan gives 12 years too, but South Korea only gives 10, with no extra market lockup. The U.S. stands out as one of the strictest markets in the world.
This delay isn’t theoretical. Take Humira, the top-selling drug in U.S. history. It got FDA approval in 2002. The first biosimilar didn’t hit the market until 2023-21 years later. Meanwhile, patients in Europe started getting cheaper versions in 2018. That’s a five-year gap where Americans paid full price while others got savings. According to I-MAK, this kind of delay cost U.S. patients over $167 billion between 2012 and 2023 just on Humira, Enbrel, and Eliquis.
Why does it take so long? The patent dance
It’s not just the 12-year clock. There’s another layer: the patent dance. This isn’t a literal dance. It’s a legal process baked into the BPCIA that forces biosimilar makers and drugmakers to exchange confidential information and negotiate which patents to fight over.
Here’s how it works: Once the FDA accepts a biosimilar application, the applicant must send the original drugmaker a copy of their entire filing. The drugmaker then has 60 days to list every patent they think might be violated. The biosimilar maker gets another 60 days to respond, explaining why each patent doesn’t apply. Then both sides have 15 days to pick which patents to litigate right away.
It sounds fair. But in practice, it’s been weaponized. Companies like AbbVie piled over 160 patents on Humira-even though the core patent expired in 2016. That created a legal maze. Each patent fight could delay a biosimilar by months or years. Courts got flooded with cases. By 2020, 87% of all BPCIA lawsuits involved more than one patent claim, according to Duke University law professor Arti Rai.
Some biosimilar makers tried skipping the dance. In the landmark Amgen v. Sandoz case, the Supreme Court ruled in 2017 that the patent dance was optional. But most companies still play along-not because they want to, but because they’re afraid of getting sued later if they don’t.

Who wins? Who loses?
The drugmakers win. They keep prices high. They extend profits. They fight every biosimilar in court. The Biotechnology Innovation Organization (BIO), which represents big pharma, says the 12-year exclusivity is necessary to fund innovation. They argue that without it, no one would spend hundreds of millions developing a complex biologic.
But patients and providers lose. Dr. Peter Bach from Memorial Sloan Kettering found that U.S. cancer patients pay up to 300% more for the same biologic than patients in Europe. Pharmacists say 63% of their patients have skipped doses or stopped treatment entirely because they couldn’t afford the drug. The Arthritis Foundation documented cases where Humira’s price jumped 470% between 2012 and 2022-while European prices stayed flat after biosimilars arrived.
Even the healthcare system loses. The Congressional Budget Office estimated that if biosimilars entered faster, the U.S. could save $158 billion over the next decade. Under current rules? Only $71 billion. That’s a gap of $87 billion-money that could pay for screenings, mental health care, or insulin for millions.
The biosimilar void: 118 drugs, almost no competition
Here’s the scary part: Even with patents expiring, biosimilars aren’t coming fast enough. Between 2025 and 2034, 118 biologics will lose patent protection. That’s a $234 billion market. But according to IQVIA, only 12 of those drugs have any biosimilar in development.
Why? Three big reasons:
- Cost: Developing a biosimilar takes 5 to 9 years and costs over $100 million. For complex drugs like antibody-drug conjugates or gene therapies, it can hit $250 million. That’s 50 to 100 times more than making a generic pill.
- Complexity: Biologics are made from living cells, not chemicals. Even tiny changes in manufacturing can affect safety. That means biosimilar makers need massive clinical trials to prove they’re nearly identical. It’s not like copying a tablet.
- Orphan drugs: 64% of the expiring biologics treat rare diseases. These drugs have small patient pools, so companies don’t see enough profit to justify the investment. Only one orphan biologic-eculizumab-has a biosimilar in the pipeline. Eighty-eight percent of orphan biologics have none.
The FDA has tried to help. Their 2022 Biosimilars Action Plan promised to improve communication, speed up reviews, and support competition. But so far, only 38 biosimilars have been approved in the U.S. since 2015. Europe has 88. The gap isn’t closing.

What’s next? Will biosimilars finally arrive?
There’s hope. The first wave of big biologics-Humira, Enbrel, Rituxan-is finally opening up. More companies are entering the space. Pfizer, Samsung Bioepis, and Amgen are all pushing forward. But the real test is what happens after 2025.
Complex drugs like CAR-T cell therapies, bispecific antibodies, and next-gen biologics are about to lose patent protection. None have biosimilars in development. If nothing changes, patients will pay sky-high prices for these life-saving treatments for years longer than necessary.
Legislators have tried to fix this. The Biosimilars User Fee Act of 2022 aimed to reduce approval delays. It died in committee. Without policy changes, the system will keep favoring patent lawyers over patients.
The truth? Biosimilars aren’t the problem. The system is. The 12-year exclusivity period, the patent thickets, the lack of incentives for complex drugs-all of it adds up to a bottleneck that hurts patients, strains insurers, and wastes billions. Europe figured out how to make biosimilars work. The U.S. hasn’t. And until it does, millions will keep paying more than they should for the medicines they need.
How long must a biologic be on the market before a biosimilar can be approved in the U.S.?
A biosimilar cannot be approved by the FDA until 12 years after the original biologic received its first FDA approval. This is called the 12-year exclusivity period under the BPCIA. Biosimilar manufacturers can submit their applications after 4 years, but the FDA cannot grant approval until the full 12 years have passed.
What’s the difference between a biosimilar and a generic drug?
Generics are exact copies of small-molecule drugs made from chemicals. Biosimilars are highly similar versions of complex biologic drugs made from living cells. Because biologics are much more complex, biosimilars can’t be identical-they’re "highly similar" with no clinically meaningful differences in safety or effectiveness. That’s why biosimilars require more testing and cost far more to develop than generics.
Why are biosimilars so expensive to develop?
Biologics are made using living organisms like bacteria or yeast, and their manufacturing process is extremely sensitive. Even tiny changes in temperature, pH, or equipment can alter the final product. Developing a biosimilar requires years of testing, thousands of lab experiments, and large clinical trials to prove similarity. The average cost is over $100 million, and for complex drugs like cell therapies, it can exceed $250 million.
Do patents delay biosimilar entry even after exclusivity ends?
Yes. Even after the 12-year exclusivity period ends, innovator companies often use "patent thickets"-hundreds of secondary patents on delivery methods, formulations, or uses-to block biosimilars through litigation. For example, AbbVie held over 160 patents on Humira, many filed after the original patent expired, to delay competition.
Why are so few biosimilars being developed for rare diseases?
Most orphan biologics treat very small patient populations, so the potential sales are low. With development costs often exceeding $250 million, companies see little financial incentive to invest. As a result, 88% of expiring biologics with orphan indications have no biosimilar in development, even though patients desperately need affordable options.
How does the U.S. compare to other countries on biosimilar access?
The U.S. has the longest exclusivity period at 12 years. The EU offers 10 years of data protection plus 1 year of market exclusivity (11 total). Japan offers 8 years of data protection and 4 years of market exclusivity (12 total), while South Korea offers 10 years of data protection with no extra market exclusivity. As a result, biosimilars enter markets like Europe and South Korea years earlier than in the U.S., leading to lower drug prices and faster patient access.
What can be done?
Change isn’t impossible. The European model shows biosimilars can succeed: 72% of the market for biologics with biosimilar competition is now made up of biosimilars. That’s because Europe has clearer rules, faster approvals, and stronger incentives for pharmacies and doctors to prescribe them.
The U.S. needs three things: First, shorten the exclusivity period to 10 years. Second, limit patent thickets by requiring patent holders to prove each patent is truly novel and relevant. Third, create financial incentives-like tax credits or guaranteed contracts-for companies developing biosimilars for rare diseases.
Until then, patients will keep paying more. And the system will keep working for lawyers and drugmakers-not for the people who need the medicine.






Joy F
January 3, 2026 AT 21:08Let’s be real - this 12-year exclusivity is just corporate welfare dressed up as innovation. Pharma doesn’t need 12 years to recoup R&D; they need 12 years to maximize shareholder dividends while patients go bankrupt. The ‘patent dance’? More like a tango with lawyers where the only winner is the billing department. And don’t get me started on Humira’s 160 patents - that’s not intellectual property, that’s legal graffiti on the public’s healthcare.
Europe got it right: 11 years, then boom - biosimilars flood in, prices drop 80%, and people actually get treated. Meanwhile, we’re still paying $10,000 a shot because some CEO thinks his patent portfolio is sacred. This isn’t capitalism. It’s feudalism with a FDA stamp.
Palesa Makuru
January 5, 2026 AT 01:57Y’all act like this is new? In South Africa, we’ve been watching this play out for decades - life-saving meds locked behind patents while people die waiting. The U.S. isn’t special, it’s just louder. You think your healthcare system is ‘advanced’? It’s just optimized for profit, not people. I’ve seen mothers choose between insulin and rent. No one’s dancing here - they’re just begging.
And don’t give me that ‘but innovation!’ crap. Innovation doesn’t need 12 years of monopoly to exist. It needs funding - not legal armor. If you can’t innovate without a 12-year cash grab, maybe you’re not an innovator. You’re a rent-seeker.
Lori Jackson
January 5, 2026 AT 12:24Oh please. The real tragedy here isn’t the 12-year window - it’s that we’ve normalized this level of corporate predation as ‘the market.’ Biosimilars aren’t ‘cheap knockoffs’ - they’re the only ethical alternative to pharmaceutical extortion. And yet, we’re still letting Big Pharma dictate terms because we’re too numb to care anymore.
It’s not about science. It’s about power. They don’t fear competition - they fear accountability. That’s why they stack patents like Jenga blocks. That’s why they sue anyone who dares to enter. That’s why patients are collateral damage in a game where the board is rigged.
And the FDA? Complicit. They approve biosimilars but don’t enforce competition. They’re not regulators - they’re gatekeepers for billionaire interests.
Sarah Little
January 7, 2026 AT 04:37Just wanted to point out that the BPCIA’s patent dance was supposed to be a compromise - but it’s been weaponized because there’s zero enforcement on patent quality. If the USPTO had actually rejected frivolous patents like ‘delivery method for a needle’ or ‘blue packaging,’ we wouldn’t be here. But they didn’t. So now we’re stuck in litigation purgatory.
Also, the cost of biosimilar development isn’t just technical - it’s psychological. Why invest $200M when you know the innovator will drag you through 10 years of lawsuits? Rational actors don’t do that. Only martyrs do. And we’re asking patients to be the martyrs.
innocent massawe
January 8, 2026 AT 22:48erica yabut
January 10, 2026 AT 16:41Let me paint you a picture: Imagine if Netflix owned every movie ever made and charged $500/month to watch ‘The Godfather’ - because they ‘invented’ the concept of storytelling. That’s what’s happening here. Biologics aren’t inventions - they’re discoveries. And now we’re paying for the privilege of accessing human biology.
The 12-year clock? It’s not science. It’s spectacle. A theater where lobbyists write the script, judges play the chorus, and patients are the silent extras. Europe? They turned biosimilars into a public health win. We turned it into a shareholder dividend.
And orphan drugs? 88% with no biosimilars? That’s not market failure. That’s moral failure. You don’t get to call yourself a civilization when you let children die because the math doesn’t add up for investors.
Vincent Sunio
January 12, 2026 AT 01:08There is a fundamental misunderstanding in the post regarding the economic rationale for the 12-year exclusivity period. Biologics represent an order-of-magnitude increase in development complexity compared to small-molecule pharmaceuticals. The marginal cost of replication is not linear. Clinical trials for biosimilars require statistically significant equivalence testing across multiple endpoints - including immunogenicity, pharmacokinetics, and long-term safety profiles.
Moreover, the assertion that patent thickets are inherently abusive ignores the legitimate need for layered intellectual property protection in a field where minor modifications can yield clinically distinct outcomes. The BPCIA was crafted with precision by Congress in consultation with the NIH, FDA, and industry stakeholders.
Comparisons to Europe are misleading. The EMA operates under a different regulatory philosophy, one that prioritizes speed over rigor. The U.S. system, while slower, ensures greater patient safety. The $167 billion figure cited is speculative and fails to account for reinvestment in R&D.
Finally, the notion that policy change is imminent is premature. Legislative action requires consensus. The Biosimilars User Fee Act failed because it lacked stakeholder alignment - not because of corporate malice.
Haley Parizo
January 12, 2026 AT 08:06You think this is about patents? Nah. This is about control. The entire system is designed to make sure that only one group - the ones who own the patents, the labs, the lobbyists - ever gets to decide who lives and who dies. Biosimilars aren’t just drugs. They’re rebellion. And rebellion? They crush it with lawsuits, delays, and fear.
Look at the numbers: 118 biologics expiring by 2034. Only 12 biosimilars in development. That’s not a market gap. That’s a massacre. And the worst part? We’re all just watching. We scroll, we share, we sigh - then we pay the bill.
Europe didn’t win because they’re smarter. They won because they stopped treating medicine like a luxury item. Here? We treat it like a stock ticker. And patients? We treat them like data points in a spreadsheet.
They say innovation needs protection. But what’s really being protected? The right to profit - not the right to heal.
Angela Fisher
January 12, 2026 AT 17:10Okay, but what if the 12-year thing is actually a cover-up? What if the real reason biosimilars don’t come out is because the FDA and Big Pharma are in on it together? Like, they’re using patents as an excuse to hide the fact that they’re secretly poisoning the biosimilar manufacturing process with trace contaminants to make them look unsafe?
I read this one guy on TruthFeed who said the FDA has a secret database of biosimilars that were proven safe but buried because they’d make insulin too cheap. And get this - they’re using AI to predict which patients will get sick from high drug prices and then target them with ads for more expensive meds.
And don’t even get me started on the Illuminati. They own 78% of the biotech patents. I saw a video where a guy in a lab coat whispered ‘12-year rule’ into a mirror and his reflection said ‘PAY MORE.’
They’re not stopping biosimilars because of patents. They’re stopping them because they want us to be dependent. And if you think this is just about money, you’re not thinking deep enough. This is about control. Total control. And they’re using your insulin to do it.
Joy F
January 14, 2026 AT 10:37^ this is why we’re doomed. Someone actually believes the FDA is hiding biosimilars because of Illuminati mind control. Meanwhile, the real issue - patent thickets, lobbying, 12-year monopolies - is still sitting there, untouched, while we argue about mirrors and AI targeting.
We’re not just failing healthcare. We’re failing critical thinking.